What Information Does a Solubility Table Give
This is the solubility table that is available for use within ALEKS. The table below provides information on the variation of solubility of different substances mostly inorganic compounds in water with temperature at one atmosphere pressureUnits of solubility are given in grams per 100 millilitres of water g100 mL unless shown otherwise.

Usp Solubility Criteria Download Table
Its possibly easier to memorise the insoluble substances than the soluble ones.
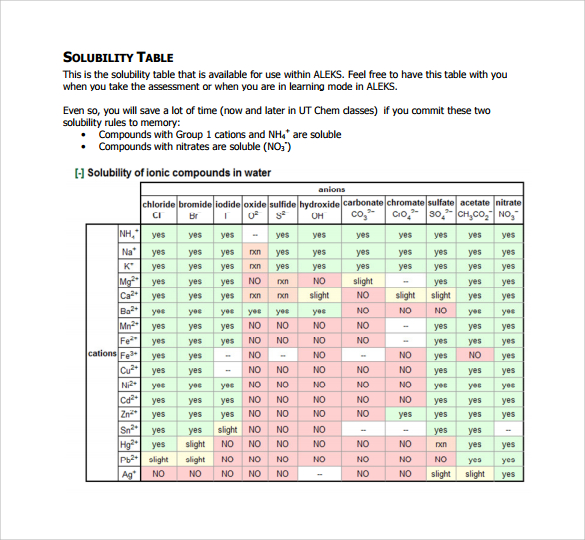
. Feel free to have this table with you when you take the assessment or when you are in learning mode in ALEKS. Under certain conditions equilibrium solubility may be exceeded to give a so-called supersaturated solution. The solubility of alkaline earth metal com-pounds decreases as you go down the column in the periodic table ie solubility decreases as the atomic mass of the alkaline A1 B1 C1 D1 A2 B2 C2 D2 A3 B3 C3 D3 A4 B4 C4 D4 A5 B5 C5 D5 A6 B6 C6 D6 IO 3 SO 4 2 C 2O 4 CO 3 2 control MgCl 2 CaCl.
For any drug its solubility is an important physical chemical property. Compounds with Group 1 cations and NH 4. Given enough time even large particles will eventually dissolve.
USP and BP solubility criteria. When mixed with other ions they can become precipitates or remain aqueous. For example table salt has avery high water solubility while glass has a very low watersolubility.
Just only in terms of quantification and have defined the criteria as given in Table 1 7 8. When mixed with other ions they can become precipitates or remain aqueous. When a solute is mixed with a solvent there are three possible outcomes.
In general I tell students to start by memorising the 5 insoluble Chlorides and Sulfates. It tells which compounds will dissolve in water. It tells which compounds will dissolve in water.
12 20 carbon disulfide CS 2. For many solids dissolved in liquid water solubility tends to correspond with increasing temperature. If there is more solute than is able to be dissolved the excess separates from the.
What information does a solubility table give. Acetone CH 3 2 CO. Soluble - soluble more than 1g per 100g of water low - low solubility 001g to 1g per 100g of water insoluble - insoluble less than 001g per 100g of water.
A solubility chart with a list of ions. From the solubility information in the table below prepare a solubility curve graph for BaCl2. A new and reliable information on the solubility of salts acids and bases.
Solubility does not depend on particle size. Solubility does not also depend on particle size or other. Temperature and the solubility of salts.
N denotes number of moles of gas. 983 15 propanol CH 3 2 CHOH 50. And solubility is defined g100ml H2O.
Solubility and Solids. What information does a solubility table give. R denotes gas constant.
The term water solubility means the degree to which a givensubstance will dissolve in water. Heavier salts do not have a higher solubility in fact many of the heavy metal salts are insoluble. Once you can recite that list off the top of your head then add in most carbonates.
Factors Affecting Solubility Temperature. Create a graph of solubility versus temperature using the data in Table 1. When mixed with other ions they can become precipitates or remain aqueous.
Solubility of Common inorganic compounds grams solute per 100 mL of water Compound. Interactive and user-friendly interface. Design experiments in the virtual lab to answer the follow questions.
Solubility is impaired by two direct factors. The possibility of studying the gaming table. A solubility chart with a list of ions.
7 0 122 10 218 20 294 25 397 30 488 40 473 50 464 60 451 80 447 100 427 120 393 140 glycerol HOCH 2 CHOHCH 2 OH. The substances are listed in alphabetical order. We use Flash technology.
The solubility should be grams100grams H2O as the volume of water changes with temperature. Temperature 0C 20C 40C 60C 80C 100C g BaCl2 100 g H20. You may have noticed that you can dissolve more sugar or table salt in hot water than in cold water.
A solubility chart with a list of ions. It tells which compounds react with oxygen. The values at STP will be.
It tells which compounds will dissolve in water What tells which compounds are soluble insoluble or slightly soluble. It tells which metals will replace other metals. B What is the solubility of BaCl2 at 50C.
Solubility is the phenomenon of dissolution of solute in the solvent to give a homogeneous system. 316 357 407 466 526 588 Outline a As the temperature increases does the solubility of BaCl2 in water increase or decrease. Solventless water H 2 O.
The solubility of both solids and gases is affected by temperature but pressure only influences the solubility of gases. The solubility pattern observed for alkaline earth metal compounds is shown below. As the temperature increases does the solubility of BaCl_2 in water increase or decrease.
The solubility table shows you a list of salts that are soluble and insoluble. It is not yours to decide which is right. T25 C273 K 29815K.
Even so you will save a lot of time now and later in UT Chem classes if you commit these two solubility rules to memory. Using the graph find the solubility of BaCl_2 at 50 degree C. From the solubility information in the table below prepare a solubility curve graph for BaCl_2.
This is because the solubility of these substances increases with temperature. If you placed a beaker containing a saturated solution of BaCl_2 at 80 degree C into a refrigerator at. 215 297 ethanol C 2 H 5 OH 50.
The following chart shows the solubility of multiple independent and various compounds in water at a pressure of 1 atm and at room temperature approx. If the solution has less solute than the maximum amount it is able to dissolve the solubility it is a dilute solutionIf the amount of solute is exactly the same as the solubility it is saturated. The following chart shows the solubility of multiple independent and various compounds in water at a pressure of 1 atm and at room temperature approx.
It is one of the important parameters that will help you to achieve the desired concentration of drugs in systemic circulation for the most anticipated pharmacological response. The following chart shows the solubility of multiple independent and various compounds in water at a pressure of 1 atm and at room temperature approx. It tells which reactants are in the gaseous state.
The solubility of a given solute in a given solvent typically depends on temperature. Solubility is the overall volume of a material that can melt at a certain temperature in a specified amount of solvent. Solventless ethanol C 2 H 5 OH 40.
Solubility Table From A Chemistry Book Download Scientific Diagram

Solubility Table Easy Hard Science
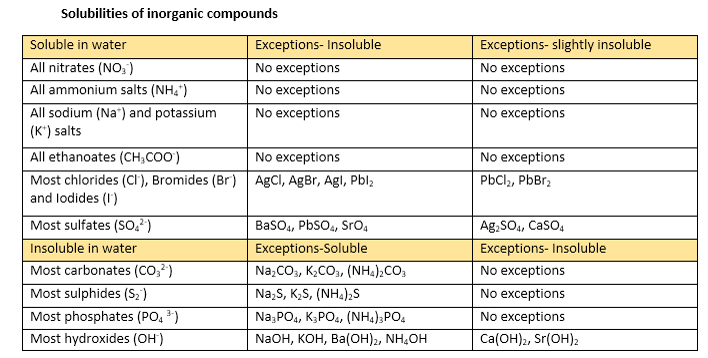
Solubility And Dissolving Vce Chemistry

Usp And Bp Solubility Criteria Download Table
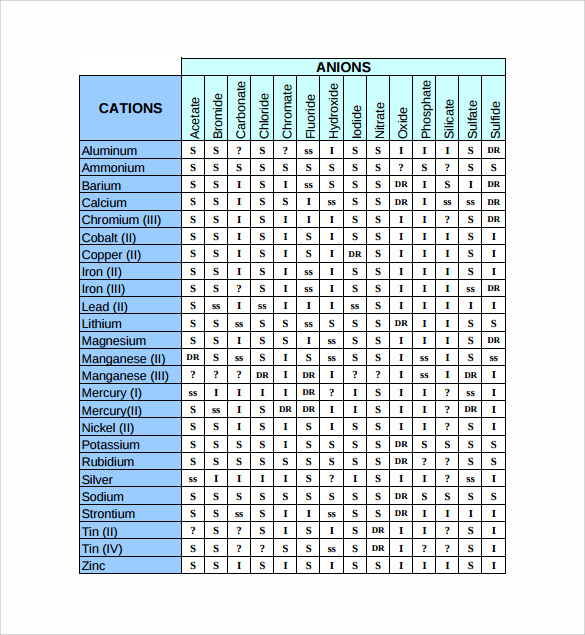
Free 8 Sample Solubility Chart Templates In Pdf Ms Word Excel

Ip Solubility Criteria Download Table

Solubility Table Easy Hard Science

Solutions Why Are Entries Missing On A Solubility Data Chart For Ionic Compounds Chemistry Stack Exchange

Solubility Rules 2 7 1 Edexcel Igcse Chemistry Revision Notes 2019 Save My Exams
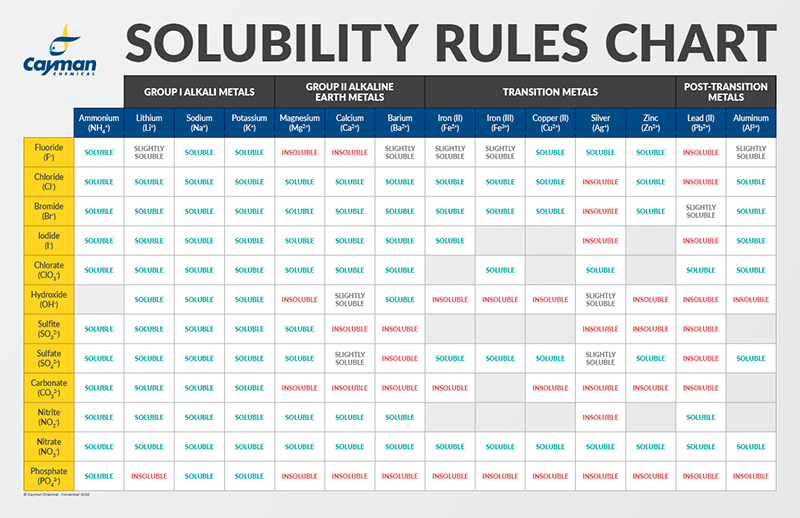
Solubility Factors When Choosing A Solvent News Announcements Cayman Chemical

How To Use The Solubility Table Chart Youtube

Solubility Introduction To Chemistry

Free 8 Sample Solubility Chart Templates In Pdf Ms Word Excel

Is Caco3 Soluble In Water Techiescientist

Solubility Table From A Chemistry Book Download Scientific Diagram
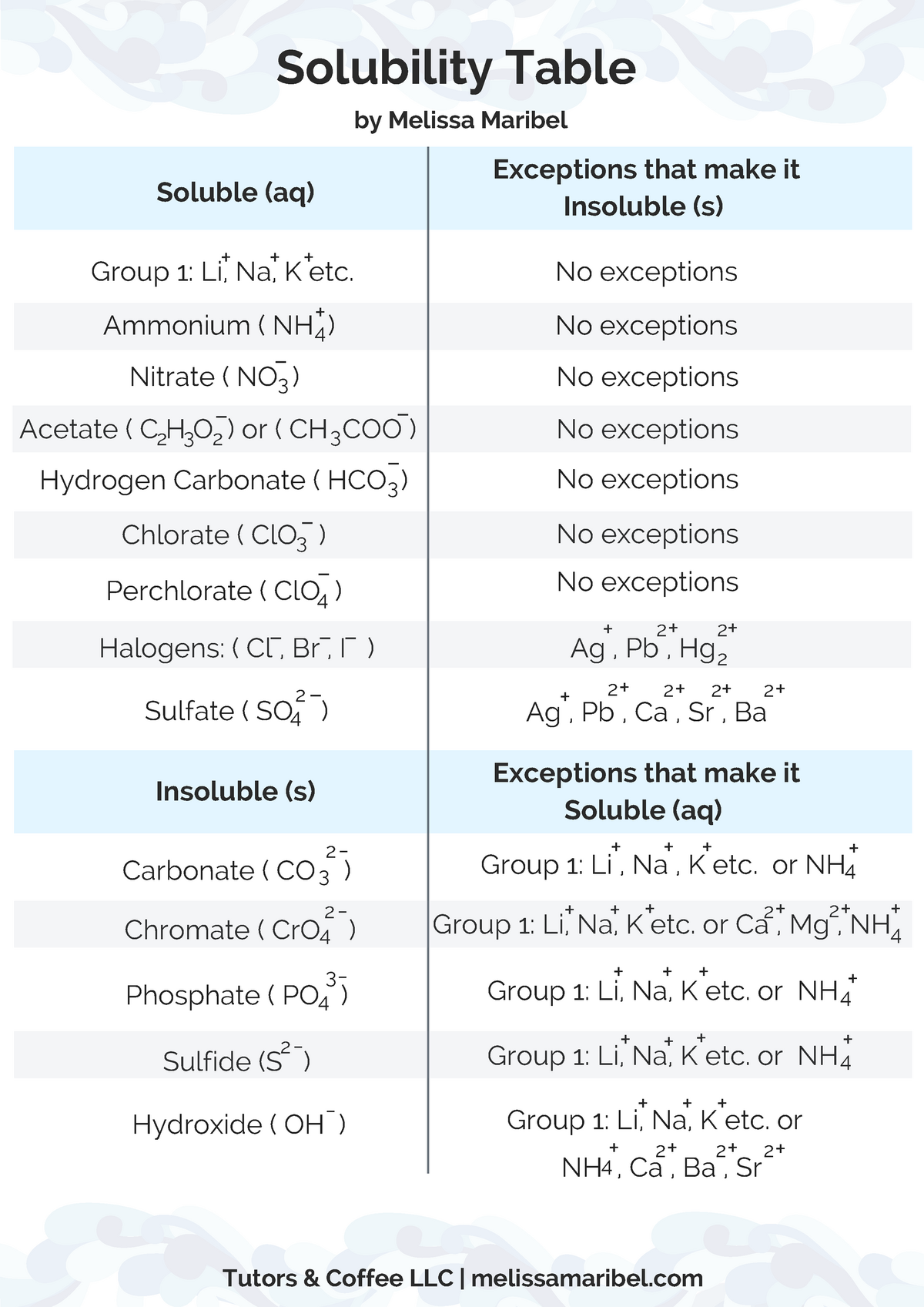
Solubility Table Ammonium Nh Nitrate No 3 Acetate C2h3o 2 Or Ch 3 Coo Studocu
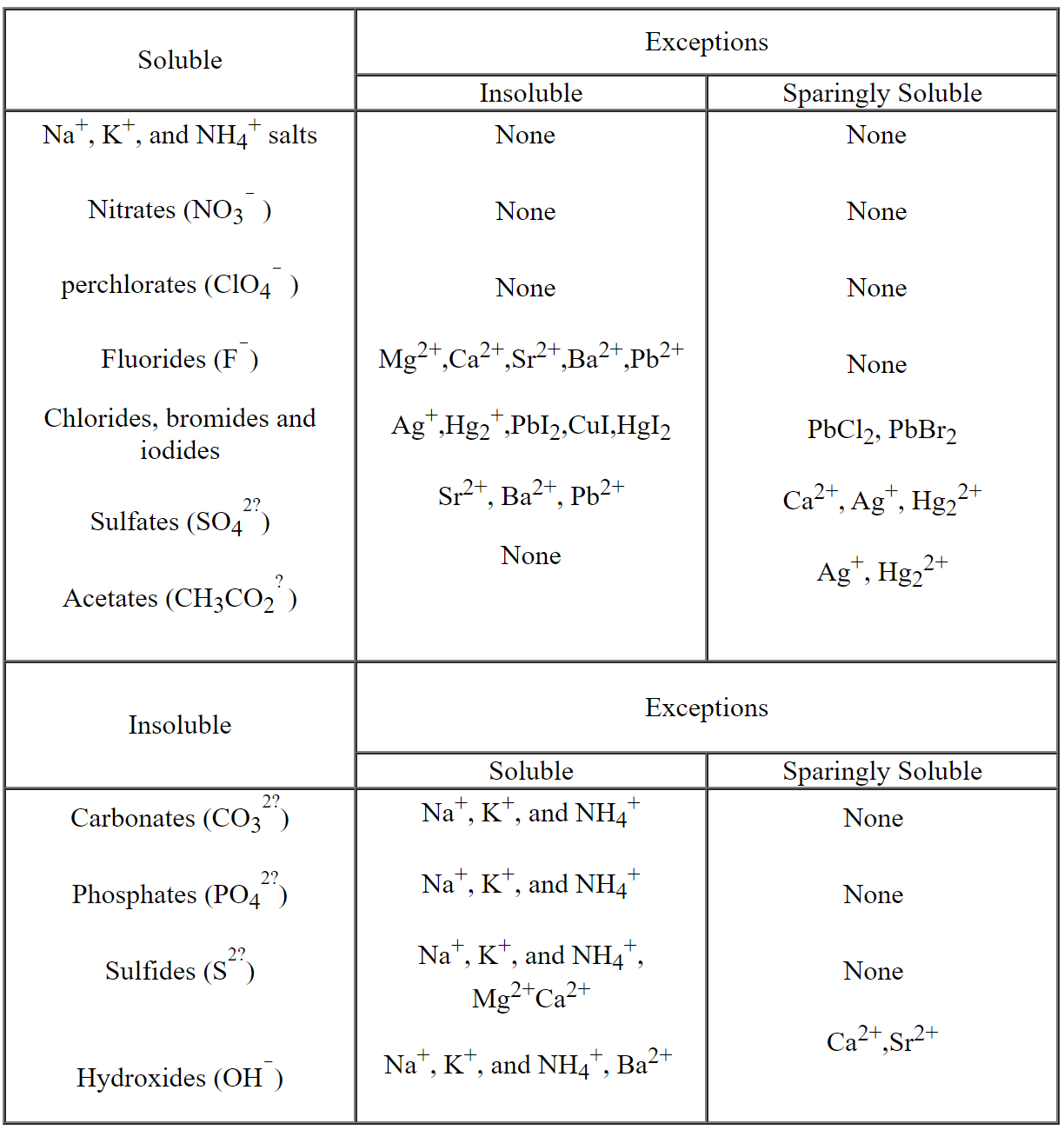
Appendix 7 Table Of Solubilities First Year General Chemistry

Comments
Post a Comment